- The proper extent of quarantine and isolation as measures against epidemics was the subject of regular debate through the Nineteenth Century. This article gives a good account of how those debates played out in New York and Massachusetts [Susan Wade Peabody, Journal of Infectious Diseases, February 1909]
- “Without legal immunity, colleges … that reopen will no doubt face suits from those who get sick.” [Jennifer Braceras] More: Why Mitch Daniels, president of Purdue, is determined to get the university reopened this fall for in-person instruction [Washington Post]
- Litigation ahead over question of how big a refund colleges may owe students of unused dormitory space [Jessica Goodman, AZFamily.com, Michael Abramowicz and Caprice Roberts] Law firm files 18 class actions against colleges and universities demanding refund on grounds that online academic program not as good as the in-person instruction it replaced [Susan Adams, Forbes]
- “New Jersey Attorney General: Employers May Have to Restrict Employees’ Saying ‘Chinese Virus'” [Eugene Volokh] First Amendment protects the right to voice irresponsible and wrong opinions, and judge should toss pressure group’s attempt to silence Fox News commentary on virus [Malathi Nayak, Bloomberg]
- “Local governments in Nevada suspend public-sector union contracts in response to COVID-19” [Jerrick Adams, Center Square]
- When does the coronavirus pandemic excuse performance of a commercial contract? [Eugene Volokh first and second posts]
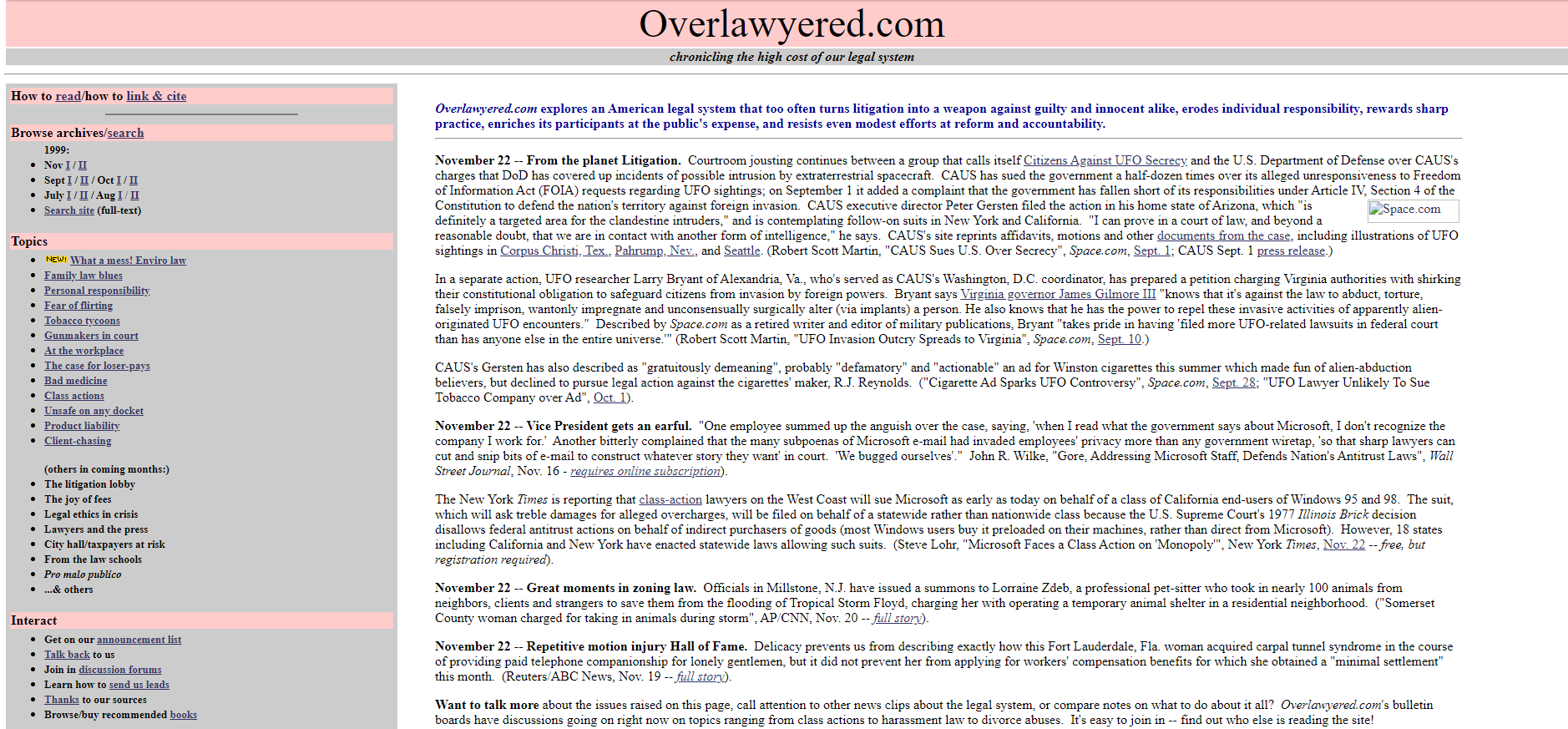
20+ years of changing blog design at Overlawyered
Longtime reader Jim Dedman reminds us of what this blog looked like in 1999:
Requiescat in pace, @Overlawyered (1999 – 2020). Thanks again for everything, @WalterOlson! pic.twitter.com/1UDw7WUCKh
— Jim Dedman (@JimDedman) May 21, 2020
As I noted when the blog turned 20:
Internet Archive’s first snapshot of the front page was taken Oct. 7, 1999, and featured the pink-and-grey color scheme that the site was to retain for many years. (Plus a webring — does anyone remember those? — an articles library, a discussion forum other than comments, and many other features since discontinued.) You can see the archives for the first half of July 1999 in Internet Archive form here.
In the site’s early years, there was no blogging software, which meant I hand-inserted every link one by one with HTML (I think Glenn Reynolds once described this method as “baked on clay tablets”). I had to make design decisions pretty much from scratch too. This resulted in a lot of homemade effects, from typeface choices to that notorious pink and gray color scheme, which was widely disliked but did make the site distinctive. Later, I moved the site onto the Movable Type and eventually WordPress blogging systems. In time, skillfully designed themes and templates became available that gave it a more professional look.
I tried to make the site a reasonably early adopter of technical innovations, but many of those were blind alleys. For example, before tags came along I lavished an inordinate amount of effort on something called categories. Blogrolls were a big social thing and source of traffic — we still have one, frozen in amber — until eventually they weren’t, after more people had begun to discover sites like ours through search and social media.
When the Cato Institute began publishing the site 10 years ago, it took over the back-end functions to my immense gratitude (thanks, Jeremy Kolassa and colleagues). This made a difference especially when WordPress would upgrade to a new version, something that used to absorb several days’ worth of my attention and often went wrong owing to my inadequacies as a techie.
One consistent theme over these nearly 21 years is the importance I’ve placed on the site’s archives. They were often the jumping-off point for my own later research into a topic, and I knew they reached a lot of journalists and scholars who would stumble across our coverage of some legal controversy from five or fifteen years earlier. Making the archives something intelligible and usable, rather than a mere jumble, could pose a special challenge whenever software upgrades altered the presentation of the site. One of the questions I’ve gotten most often in recent days is whether the archives will stay up, and I have confidence that Cato will do an excellent job on that.
Here’s another image (click to enlarge) of what the top of the site’s front page looked like in late 1999:
Longtime and other readers are welcome to share recollections of how the site used to look and work.
Reopen lawsuits, and their mixed outcomes
Lawsuits have been filed in many states challenging governors’ and mayors’ public health orders arising from the COVID-19 outbreak. I’m in the Frederick News-Post with a guest opinion piece on a judge’s rejection of the (unusually weak) Reopen suit in Maryland. Excerpts:
In some other states, challengers have won rulings striking down at least some portions of state stay-home orders. But this suit’s claims failed all down the line, and here’s why….
In Wisconsin, Oregon, and Ohio, challengers were able to convince judges that governors overstepped the authority granted under state emergency laws, which may require, for example, legislative say-so for an emergency order’s extension. But Maryland grants its governor broader power than many other states, one good reason being that ours is not a year-round legislature. The General Assembly has been adjourned for weeks and is not going to reconvene in Annapolis every 30 days — in the middle of a pandemic! — to give thumbs up or down on each Hogan order. Nor should it have to. The judge found Hogan had not overstepped Maryland law….
In some states, challengers have successfully argued that governors’ orders were too restrictive toward churches. Those claims failed here too.
Under the relevant standard, articulated by the late Justice Antonin Scalia in a 1990 Supreme Court opinion, neutral and general laws that burden religion do not violate the U.S. Constitution so long as 1) they are not improperly motivated by a wish to restrict religion, and 2) they do not arbitrarily restrict religious activity when genuinely similar non-religious activity is permitted. This court, like other federal courts, rejected the argument that if stores are to stay open to sell plywood or soft drinks, all other gatherings must be permitted as well. As the judge pointed out, the federal government’s own guidelines designate sale of food and cleaning supplies as essential. And shop-and-leave arrangements can be rationally distinguished from gatherings whose whole point is to congregate closely for a lengthy period. (Religious gatherings have been an important source of outbreaks both in the U.S. and abroad.)
Some other perspectives on Reopen litigation: Bonnie Kristian/The Week quoting me, Ilya Shapiro, Jacob Sullum, Larry Salzman/PLF; Lawfare resources on state emergency authorities and quarantine/isolation laws; NCSL on state law authority.
More mentions on Overlawyered’s adjournment
The nicest single thing anyone says about me this week might turn out to be from Bob Ambrogi at Legal Sites: “Let’s retire jersey number one.” Thanks! Or it might be one of the kind things Scott Greenfield says here. Here’s what I wrote in response to Scott:
Thank you for those kind words. As you guessed, my choice of “adjourned” was mostly because of its legal flavor, but also signaled that I’m not retiring or going away. I like blogging! I continue to post at the group blog Cato at Liberty and at my low-frequency Maryland politics blog Free State Notes, and I might be tempted at some point to try a blog project with a limited subject and duration (several people have suggested I blog the coronavirus crisis). What I can’t go on shouldering is the commitment needed for an open-ended, daily-or-more-so, individual blog. You yourself set an outstanding example of how to go about doing that.
More coverage of my resolution to hang it up May 31: Bloomberg Law in a roundup, Eugene Volokh, Raised on Hoecakes. And: Steve Bainbridge (“one of those that inspired me to start my own blog. His site has been a must read since day one.”); Eric Turkewitz (“I’ve taken some crap over the years from other personal injury lawyers over my lauding of Olson and his site. But it was the way he did things that was important to me.”).
The required, yet also somehow forbidden, front-door temperature check
As society struggles to contain the epidemic, should businesses screen arriving customers and workers for fever using forehead temperature guns? The beauty of our legal system is that the business can get sued whichever way it decides to go. My new piece at Cato, following up on one last week.
Tom Sawyer’s funeral: the Twitter version
The fun in closing down an enterprise like Overlawyered before you “have to” is that you can be like Tom Sawyer watching the tributes at your own funeral. Sticking to Twitter for now, here are ten or so of my favorites so far. From former co-blogger Ted Frank quoting Scott Greenfield:
.@overlawyered changed the course of my career. Loved reading it, was honored to write for it (first guest post 9/3/03), and readers emailing me and @walterolson to complain about lack of effective options to deal with bad class action settlements got me thinking about solutions. https://t.co/il2luuDMhB
— tedfrank ? (@tedfrank) May 21, 2020
And leading legal bloggers:
.@overlawyered inspired so many longtime/OG bloggers including me. 20+ years of daily blogging is a truly remarkable achievement. There will never be another blogger like @walterolson ????? https://t.co/TeCkWoI9Hi pic.twitter.com/hfY3jtZ50M
— Eric Goldman (@ericgoldman) May 21, 2020
Lawblog Superman hangs up his cape.https://t.co/upF4rYlXu0
— SoiledAndOrFoiledHat (@Popehat) May 21, 2020
And reporters and journalists:
You will be SORELY missed, Walter. Above all else, you are open-minded and respectful — qualities in notably short supply. https://t.co/okx2r2RrvE
— Alison Frankel (@AlisonFrankel) May 21, 2020
Cheers, Walter. Thanks from all the reporters who shamelessly prowled @overlawyered for tips and insights. Especially me. https://t.co/UadOtJWJgu
— Joe Palazzolo (@joe_palazzolo) May 21, 2020
And from others who say their careers or education took a different path:
Walter, I owe my blogging start to reading you. From that grew my practice. From that, I gave people jobs and helped a lot of people. Your influence meant a lot to many who may never know your name.
On my own behalf, and theirs, Thank you.
— Marc J. Randazza (@marcorandazza) May 21, 2020
Overlawyered made me a better law student. https://t.co/CmzRNbuIiU
— Awakey Joe (@AwakeyJoe) May 21, 2020
Thanks for all your work on Overlawyered over the years, Walter. It was one of the blogs that inspired me, early on, to pursue a career in policy. An incredible run!
— Jarrett Dieterle (@JarrettDieterle) May 21, 2020
And some who just wanted to let off steam:
NOOOOOOOOOOOOOOOOOOOOOO https://t.co/tjUkWYkHV9
— Robert VerBruggen (@RAVerBruggen) May 21, 2020
Adjourned: Overlawyered to cease publication May 31
Dear friends and Overlawyered readers:
I’ve been considering ceasing publication of Overlawyered over the past couple of years, and the time has finally arrived. I plan to publish its final post on May 31, ten days from now.
That will leave the site just one month short of a remarkable 21-year run. That makes it the longest-running general interest law blog anyone has been able to identify. It’s one of the monuments still standing from the heyday of individual blogging on current events and public policy, a sector that bloomed after 9/11 two years into our run.
It has been a pleasure beyond compare to write it. But blogs that publish every day (and with only a few exceptions, that is what Overlawyered has managed to do for all these years) are extraordinarily time-intensive for a single author, and my time is constrained.
Be assured (if you count this as assurance!) that I am not going anywhere. I look forward to continuing my writing as a Cato senior fellow both at the excellent multi-contributor blog Cato at Liberty and at many other outlets. One reason I’m making this decision is that I’m eager to step up the pace of this other writing at a time rich in policy challenges.
Even in its early years Overlawyered had a much broader range of interests than its name might imply. It covered (and still does cover) wacky lawsuits as well as the more serious side of litigation policy but also many areas of writing interest of mine such as free speech and business regulation. Especially since it came to Cato ten years ago, it has continued to branch out into such areas as constitutional law, criminal justice policy, and state and local policy. But at heart it has always been a blog about law in America.
I’ll have more to say in coming days to recognize and thank the site’s community of readers, contributors, and guestbloggers, to talk matters of transition and what will live on in what forms (I expect the archives to be fully available for the indefinite future, thanks Cato), to reminisce, and also to do a bit of regular blog posting as the impulse strikes. Just this once, I’m leaving comments closed on this post, but they will be open on a nearby related post.
Comment thread: Overlawyered adjourned
This is a comment thread on the news we’re announcing today about the end of Overlawyered’s long run.
I’ve had much cause to appreciate our commenters over the years, who have made me laugh, question my premises, and realize when subjects were more complicated than I thought. Overlawyered’s commenters are remarkably well informed on all sorts of topics, often much more so than I am. This has served not just to amuse and entertain, but to improve and correct my own writing. For that, and for everything else, I thank you.
May 20 roundup
- Is universal access to reliably functioning electric power better or worse in countries that officially treat access to electric power as a right rather than a private good? [David R. Henderson on Burgess et al., Journal of Economic Perspectives]
- “There’s bad lawyering, and then there’s lawyering so bad that the Tenth Circuit holds the plaintiffs’ lawyer liable for $1 mil in attorneys’ fees. But that’s what you get if you ignore orders not to file ‘any more prolix, redundant, meandering pleadings or briefs.'” [Institute for Justice “Short Circuit” on Snyder v. Acord]
- 1st Circuit: Dept. of Interior broke law when it turned land owned by Mashpee Wampanoag tribe into new reservation land. Feds: okay, we’ll comply and tribe will own land in conventional form instead. Progressive Twitter: settler colonialist shock horror! [WBUR]
- “Supreme Court Agrees to Decide, What is Hacking?” [Orin Kerr on Van Buren v. U.S.]
- “The Second Circuit has upheld the awful decision by [a district court] to sanction a building owner millions of dollars for daring to paint the walls of his own building.” [Cathy Gellis, TechDirt; earlier; Visual Artists Rights Act violation found after building owner permitted graffiti installations, later painted them over]
- “Led Zeppelin wins ‘Stairway to Heaven’ copyright case” [Jonathan Stempel, Reuters]
Oops, indeed: Oklahoma judge says he mistakenly added three zeroes in opioid payout
Somehow missed blogging this when it happened last fall: “An Oklahoma judge who ordered Johnson & Johnson to pay $572 million for its role in the state’s opioid epidemic admitted in court on Tuesday that he made a $107 million math error. Judge Thad Balkman of Cleveland County said the portion of the award devoted to a treatment program for addicted babies should have been $107,683, not $107,683,000.” [Debra Cassens Weiss, ABA Journal last October; earlier here and here on Oklahoma opioids public nuisance case] Not unrelated: “A dozen law firms are set to earn nearly $160 million in contingency fees in 15 opioid settlements involving two counties in Ohio and the state of Oklahoma, according to Law.com’s review of the contracts at issue in those settlements and emails provided by government officials.” [Amanda Bronstad, Law.com]